Human IgG1 Kappa | 純化之免疫球蛋白產品 Isotype control貨號0151K-01

| 代理廠牌: |  |
| 原廠連結: | |
| 相關下載: |
同型對照(Isotype control)-Human IgG1 Kappa
同型對照(Isotype control)目的確定一抗的結合是否特異性;而不是非特異性的Fc受體或與其他蛋白的相互作用所造成的Signal。還可以用來競爭性的結合抗體,與功能阻斷抗體發揮同樣的功能。同型對照(Isotype control)應用於流氏細胞儀抗體分型control、ELISA抗體分型control、FLISA抗體分型control 、IHC抗體分型control 、IP抗體分型control 、invitro 抗體分型control 、invivo抗體分型control。使用Isotype control的原則: 同型對照/分型對照要與一抗的來源,Ig分型和標記完全一致。同型對照/分型對照(Isotype Control):使用與一抗相同種屬來源、相同亞型、相同劑量和相同的免疫球蛋白及亞型的免疫球蛋白,用於消除由於抗體非特異性結合到細胞表面而產生的背景染色。如果一抗是多抗,可以用an normal serum(與一抗相同的正常血清),此方法常在IHC染色中常被用於負對照組;假若多重螢光染色,由於螢光標記單抗的來源不同,應選用相同來源的未標記單抗作為同型對照來 調整背景染色。例子說明: 比如檢測一抗為單抗的mouse anti rat CD11b,clone OX-42 purified IgG,那麼它的isotype 是mouse IgG2a, 所以可以用purified(純化的) mouse IgG2a來做OX-42的同型對照(Isotype Control)。
Human IgG1 Kappa Isotype control-純化之免疫球蛋白產品 貨號0151k (human) | 規格0.5mg /1ml
分型對照組Human IgG1 Kappa Isotype control | 產品應用流氏細胞儀(flow cytometry)抗體分型control、ELISA抗體分型control、FLISA抗體分型control 、IHC抗體分型control 、IP(immunoprecipitation)抗體分型control 、invitro 抗體分型control 、invivo抗體分型control、Fc介導的刺激(Fc mediated stimulation)、表面等離子體共振中的固定化( immobilization in surface plasmon resonance)。
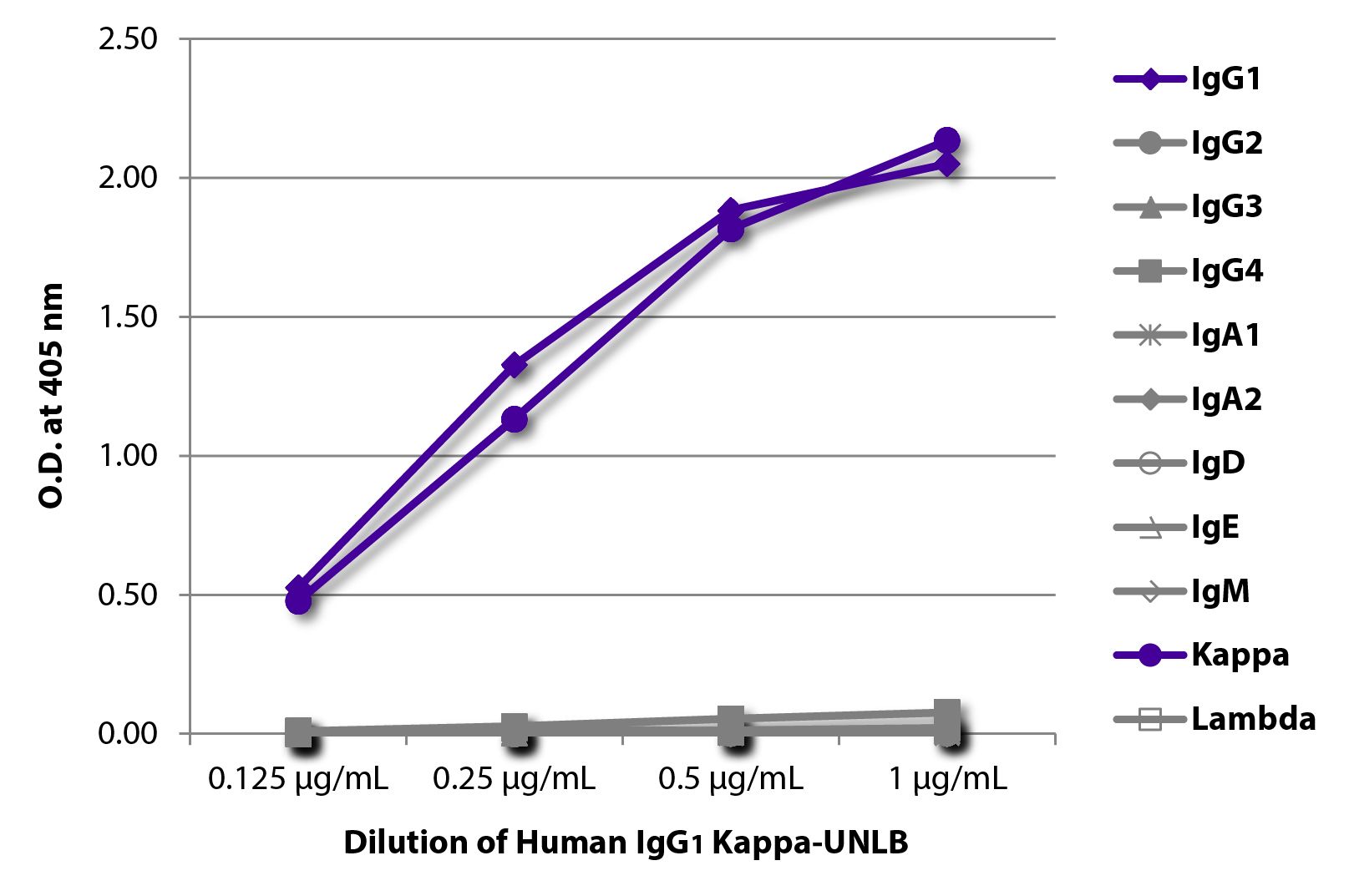
| Isotype | Human IgG1κ |
|---|---|
| Source | Human myeloma serum |
| Conjugate | UNLB (Unconjugated) |
| Buffer Formulation | Borate buffered saline, pH 8.2 |
| Clonality | Purified from myeloma serum |
| Concentration | 0.5 mg/mL |
| Volume | 1.0 mL |
| Recommended Storage | 2-8°C |
| Applications | Quality tested applications for relevant formats include – ELISA 1,2 FLISAOther referenced applications for relevant formats include – Flow Cytometry 8,9 Immunoprecipitation 3 In vitro control 4-6 In vivo control 1,7 Stimulation (Fc mediated) 10 Blocking 6 Surface Plasmon Resonance 1 |
| RRID Number | AB_2794079 |
細胞免疫專家極力推薦SouthernBiotech高品質Isotype control
美國抗體生產商Southern Biotech於1982年,由UAB細胞免疫專家Dr. Max Cooper與實驗室成員共同創立。SouthernBiotech致力於生產高品質Isotype control-免疫球蛋白系列產品,具有螢光物質(FITC、PE、APC、SPRD、PE/CY5.5、APC/CY5.5、Spectral Red、Alexa 488、Alexa 647、Pacific Blue)各種螢光標誌、另具酵素(HRP、AP)、或未標誌及Low Endotoxin/Azide Free(LE/AF)等標定。
Human分型對照組 | 提供純化之IgG1 Kappa
| Cat. No. | Product Description | Clone | Size |
| 0151K-01 | Human IgG1 Kappa-UNLB | 0.5 mg | |
| 0151K-04 | Human IgG1 Kappa-AP | 1.0 mL | |
| 0151K-05 | Human IgG1 Kappa-HRP | 1.0 mL | |
| 0151K-09 | Human IgG1 Kappa-PE | 0.1 mg | |
| 0151K-14 | Human IgG1 Kappa-LE/AF | 0.5 mg |
其它物種之分型對照組 | 提供純化及不同分型之Isotype control
| Isotype Controls | |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
1. Bock F, Onderka J, Dietrich T, Bachmann B, Kruse FE, Paschke M, et al. Bevacizumab as a potent inhibitor of inflammatory corneal angiogenesis and lymphangiogenesis. Invest Ophthalmol Vis Sci. 2007;48:2545-52. (ELISA, in vivo control, Surface plasmon resonance)
2. Zhang J, Jacobi AM, Wang T, Berlin R, Volpe BT, Diamond B. Polyreactive autoantibodies in systemic lupus erythematosus have pathogenic potential. J Autoimmun. 2009;33:270-4. (ELISA)
3. Yang G, Holl TM, Liu Y, Li Y, Lu X, Nicely NI, et al. Identification of autoantigens recognized by the 2F5 and 4E10 broadly neutralizing HIV-1 antibodies. J Exp Med. 2013;210:241-56. (IP)
4. Ackler S, Mitten MJ, Foster K, Oleksijew A, Refici M, Tahir SK, et al. The Bcl-2 inhibitor ABT-263 enhances the response of multiple chemotherapeutic regimens in hematologic tumors in vivo. Cancer Chemother Pharmacol. 2010;66:869-80. (In vitro control)
5. Li J, Jie H, Lei Y, Gildener-Leapman N, Trivedi S, Green T, et al. PD-1/SHP-2 inhibits Tc1/Th1 phenotypic responses and the activation of T cells in the tumor microenvironment. Cancer Res. 2015;75:508-18. (In vitro control)
6. Li J, Srivastava RM, Ettyreddy A, Ferris RL. Cetuximab ameliorates suppressive phenotypes of myeloid antigen presenting cells in head and neck cancer patients. J Immunother Cancer. 2015;3:54. (In vitro control, Block)
7. Woess C, Tuzlak S, Labi V, Drach M, Bertele D, Schneider P, et al. Combined loss of the BH3-only proteins Bim and Bmf restores B-cell development and function in TACI-Ig transgenic mice. Cell Death Differ. 2015;22:1477-88. (In vivo control)
8. Hotard AL, Lee S, Currier MG, Crowe JE Jr, Sakamoto K, Newcomb DC, et al. Identification of residues in the human respiratory syncytial virus fusion protein that modulate fusion activity and pathogenesis. J Virol. 2015;89:512-22. (FC)
9. Chapuy CI, Nicholson RT, Aguad MD, Chapuy B, Laubach JP, Richardson PG, et al. Resolving the daratumumab interference with blood compatibility testing. Transfusion. 2015;55:1545-54. (FC)
10. Ortiz DF, Lansing JC, Rutitzky L, Kurtagic E, Prod’homme T, Choudhury A, et al. Elucidating the interplay between IgG-Fc valency and FcγR activation for the design of immune complex inhibitors. Sci Transl Med. 2016;8:365ra158. (Stim)
11. Liu H, Chumsae C, Gaza-Bulseco G, Hurkmans K, Radziejewski CH. Ranking the susceptibility of disulfide bonds in human IgG1 antibodies by reduction, differential alkylation, and LC-MS analysis. Anal Chem. 2010;82:5219-26. (Mass spectrometry)

